StabiLink® MIS Interlaminar Spinal Fixation System
FDA Cleared

Dual Lamina

C2 Base

C3 Base

EZ Lamina
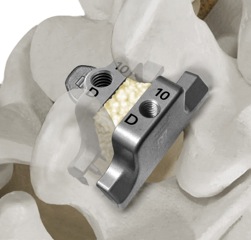
The principle benefit of the patented StabiLink® MIS Interlaminar Spinal Fixation System is that it is placed between the spinous processes, away from the neural elements including the spinal cord and spinal nerves. The implant is placed through a 2-4 cm incision using the innovative, patented PG® Precision Guided Inserter/ Compressor.
The PG®Inserter/Compressor is an All-in-One instrument that redefines ease-of-use and allows the surgeon to quickly and accurately place the StabiLink® implant with or without removal of the interspinous ligaments.
In addition, implant insertion and compression is safely achieved without the need for multiple instruments, including “bulky” compressors, reducing overall procedure time. The anterior “tray” design of the StabiLink® implant creates an ideal containment area for the maximum amount of bone graft material to optimize bony fixation between the spinous process.
- Small diameter wide-spike design (16 spikes per implant over a broad area) for increased load sharing capacity under both static and fatigue testing
- Laminar Lock Design effectively limits motion in all three planes- lateral bending, axial rotation, and flexion/extension
- Wide range of implant designs and sizes for optimum anatomical fit
- Low profile retains access to facet joints and other anatomy
- Torque controlled locking mechanism results in secure fixation

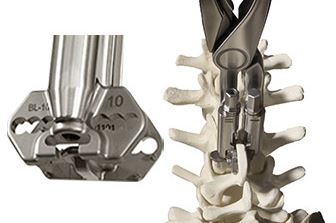
Precison Guided Inserter/Compressor
Redefines Ease-of-use
.jpg) |
|
- Interlocking Implant Insertion/Compression in One Instrument
- Automatic “3-D” alignment reduces overall procedure time
- Eliminates the need for Multiple “Bulky” Compressors, resulting in smaller incision
- Can be used with/without removal of interspinous ligaments
- Threaded Ratchet Arm for Additional Compression

.jpg)
